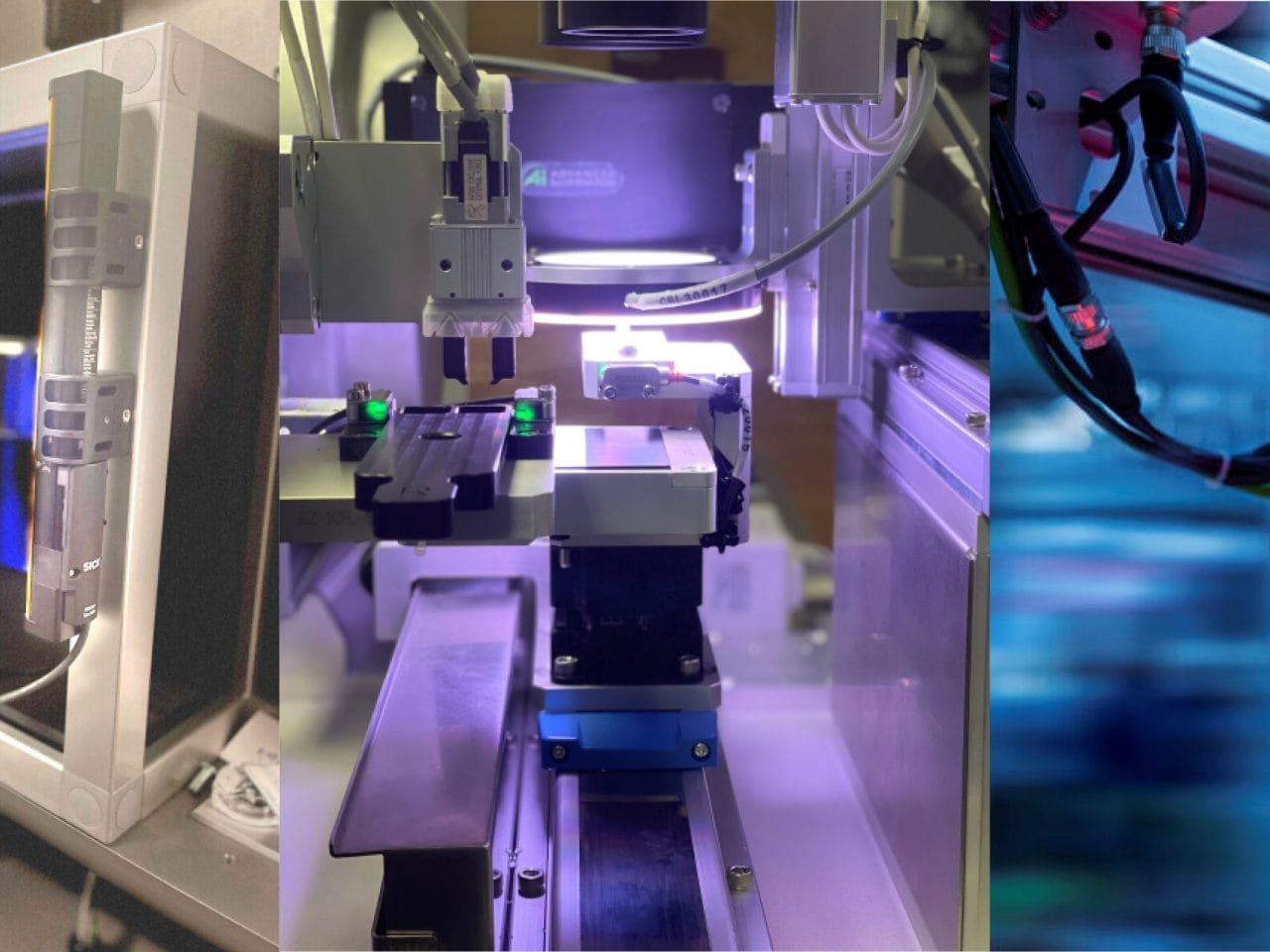
Medical device manufacturers face unforgiving standards. A single defect, labeling error, or packaging flaw can trigger costly recalls, regulatory violations, and irreparable damage to your brand. Manual inspection methods can’t keep pace with the complexity of today’s devices or the scrutiny of FDA audits and ISO 13485 requirements.
At EZ Automation Systems, we recognize that no two medical inspection problems are the same. The inspection challenges for intraocular lenses differ from those of catheters, implants, or surgical tools.
We design customized turnkey automated inspection systems designed specifically for your medical device application. Our solutions combine the optimal selection of advanced machine vision, AI-powered defect detection, and seamless integration with your existing production lines to ensure every device meets quality benchmarks before it goes to market.
Whether your priority is high-speed throughput, 98%+ detection accuracy, or audit-ready traceability, EZ Automation can design a system that fits your application’s unique requirements.
This is made possible by our multidisciplinary team of AI and computer vision engineers, robotics and automation integration specialists, and optics and imaging experts.
Our Medical Device Inspection Capabilities
- Component Verification
- Surface Defect Detection
- Dimensional Inspection
- Label and Marking Verification (OCR and Barcode Reading)
- Packaging Inspection
Why Medical Device Manufacturers Choose EZ Automation Turnkey System Integration
Turnkey System Integration
We don’t just sell cameras or software. We deliver complete inspection solutions designed to integrate with your production workflow. Our team handles everything from initial assessment and system design to installation, validation, and ongoing support.
Technology-Agnostic Approach
From camera selection and lighting engineering to software development and AI model training, we develop systems tailored to your application. Our scalable architectures are also adaptable to multiple product types and production lines.
Accelerated AI Training
Our proprietary EZ Eye machine vision platform reduces AI model training time by up to 10x compared to traditional methods. What once took weeks of engineering time now takes days, allowing your inspection system to become operational sooner and adapt quickly to product variations or new device introductions.
Regulatory Compliance Support
EZ designs systems that can support the documentation, traceability, and validation protocols called for under FDA, ISO 13485, and GMP compliance. Automated inspection logs, timestamped defect records, and cloud-based analytics can help ensure you’re always audit ready.
Medical Device Manufacturing Applications We Support
- Surgical Instruments: Ensuring surface finish, dimensional accuracy, and assembly completeness
- Implantable Devices: Critical defect detection for orthopedic, cardiovascular, and neurological implants
- Diagnostic Equipment: Component verification and calibration validation
- Drug Delivery Systems: Syringe inspection, inhaler assembly verification, autoinjector quality control
- Single-Use Devices: High-volume inspection for catheters, needles, and consumables
- Wearable Medical Devices: Assembly verification, electronics inspection, and housing integrity


Featured Solutions for Medical Device Inspection

Medical device designs are highly diverse and conventional AI training for vision inspection systems can take weeks. The EZ Eye platform allows your quality team to reduce training time for AI-driven inspections by nearly half, even if they lack deep learning expertise.

PIQuE-360 is our standalone robotic inspection platform designed for 360-degree AI-powered inspection of medical devices and components. It delivers over 95% defect detection accuracy, zero false positives after initial calibration, and significantly faster training of AI models.